Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19
MD, MSc Eduardo López-Medina, MD Pío López, MD; Isabel C Hurtado, MD, MPH, DrPH Diana M Dávalos, MD, MPhil Oscar Ramirez, MD Ernesto Martínez, MD Jesus A Díazgranados, MD José M Oñate, MD, MS Hector Chavarriaga, MD Sócrates Herrera, PhD Beatriz Parra, PhD Gerardo Libreros, MD Roberto Jaramillo, MD; Dilian Ana C Avendaño, Dilian F Toro, DrPH Miyerlandi Torres, MD Maria C Lesmes, MD Carlos A Rios, MD Isabella Caicedo
JAMA, doi:10.1001/jama.2021.3071
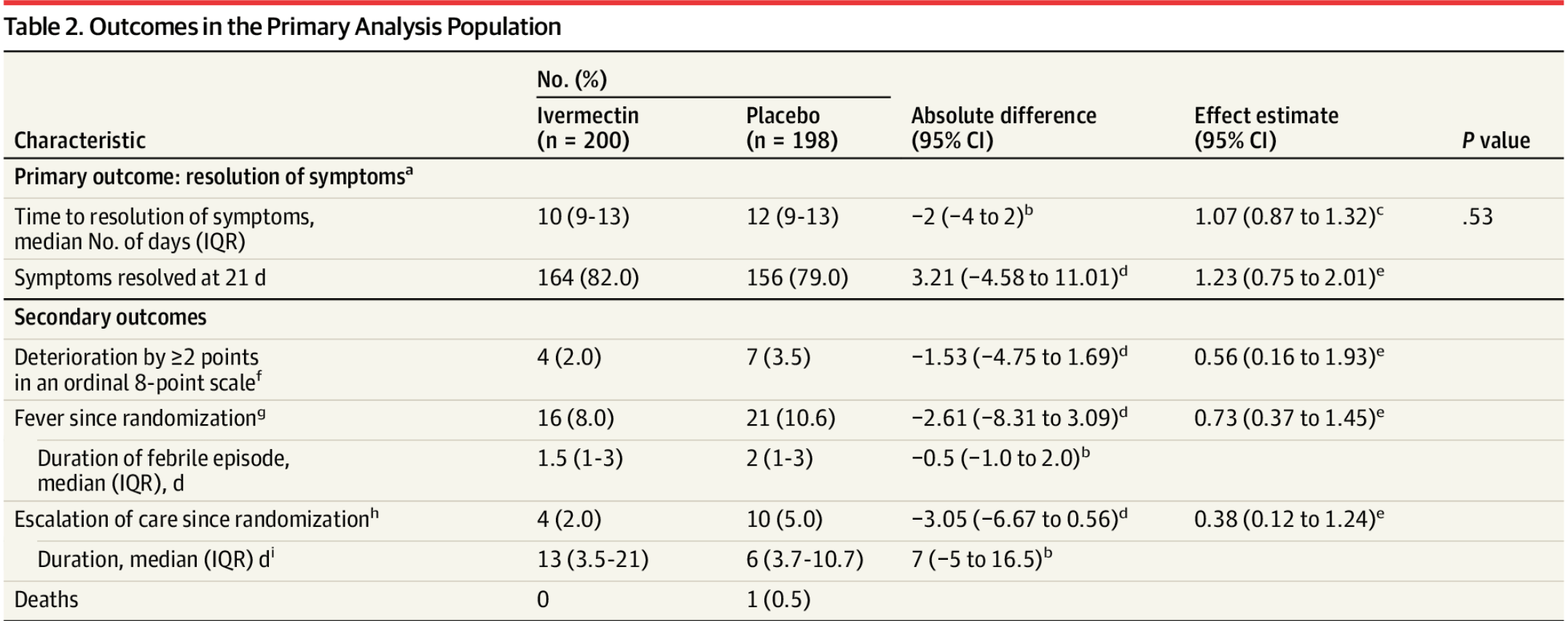
IMPORTANCE Ivermectin is widely prescribed as a potential treatment for COVID-19 despite uncertainty about its clinical benefit. OBJECTIVE To determine whether ivermectin is an efficacious treatment for mild COVID-19. DESIGN, SETTING, AND PARTICIPANTS Double-blind, randomized trial conducted at a single site in Cali, Colombia. Potential study participants were identified by simple random sampling from the state's health department electronic database of patients with symptomatic, laboratory-confirmed COVID-19 during the study period. A total of 476 adult patients with mild disease and symptoms for 7 days or fewer (at home or hospitalized) were enrolled between July 15 and November 30, 2020, and followed up through December 21, 2020. INTERVENTION Patients were randomized to receive ivermectin, 300 μg/kg of body weight per day for 5 days (n = 200) or placebo (n = 200). MAIN OUTCOMES AND MEASURES Primary outcome was time to resolution of symptoms within a 21-day follow-up period. Solicited adverse events and serious adverse events were also collected. RESULTS Among 400 patients who were randomized in the primary analysis population (median age, 37 years [interquartile range {IQR}, 29-48]; 231 women [58%]), 398 (99.5%) completed the trial. The median time to resolution of symptoms was 10 days (IQR, 9-13) in the ivermectin group compared with 12 days (IQR, 9-13) in the placebo group (hazard ratio for resolution of symptoms, 1.07 [95% CI, 0.87 to 1.32]; P = .53 by log-rank test). By day 21, 82% in the ivermectin group and 79% in the placebo group had resolved symptoms. The most common solicited adverse event was headache, reported by 104 patients (52%) given ivermectin and 111 (56%) who received placebo. The most common serious adverse event was multiorgan failure, occurring in 4 patients (2 in each group). CONCLUSION AND RELEVANCE Among adults with mild COVID-19, a 5-day course of ivermectin, compared with placebo, did not significantly improve the time to resolution of symptoms. The findings do not support the use of ivermectin for treatment of mild COVID-19, although larger trials may be needed to understand the effects of ivermectin on other clinically relevant outcomes.
Funding/Support: This study received an unrestricted grant from Centro de Estudios en Infectología Pediátrica (grant ScDi823).
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Group Information
References
Beigel, Tomashek, Dodd, ACTT-1 Study Group Members. Remdesivir for the treatment of COVID-19: final report, N Engl J Med,
doi:10.1056/NEJMoa2007764Bray, Rayner, Noël, Jans, Wagstaff, Ivermectin and COVID-19: a report in antiviral research, widespread interest, an FDA warning, two letters to the editor and the authors' responses, Antiviral Res,
doi:10.1016/j.antiviral.2020.104805Hashim, Maulood, Rasheed, Fatak, Kabah et al., Controlled randomized clinical trial on using ivermectin with doxycycline for treating COVID-19 patients in Baghdad, Iraq. medRxiv,
doi:10.1101/2020.10.26.20219345Karaca-Mandic, Georgiou, Sen, Cao, Wang et al., A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19, JAMA Intern Med,
doi:10.1056/NEJMoa2001282Mahajan, Larkins-Pettigrew, Racial demographics and COVID-19 confirmed cases and deaths: a correlational analysis of 2886 US counties, J Public Health (Oxf),
doi:10.1093/pubmed/fdaa070Mastrangelo, Pezzullo, Burghgraeve, Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug, J Antimicrob Chemother,
doi:10.1093/jac/dks147Mitjà, Corbacho-Monné, Ubals, -CoV-2 RESEARCH GROUP. Hydroxychloroquine for early treatment of adults with mild COVID-19: a randomized-controlled trial, Clin Infect Dis
Momekov, Momekova, Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: antiviral levels are not likely attainable with known dosing regimens, Biotechnol Biotechnol Equipment,
doi:10.1080/13102818.2020.1775118Niaee, Gheibi, Namdar, Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: a randomized multi-center clinical trial,
doi:10.21203/rs.3.rs-109670/v1Rajter, Sherman, Fatteh, Vogel, Sacks et al., Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ICON Study, Chest,
doi:10.1016/j.chest.2020.10.009Rodriguez, America's embrace of an unproven COVID treatment is hindering drug trials
Schmith, Zhou, Lohmer, Sanchez, Mejia-Fernandez et al., The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19, Clin Pharmacol Ther,
doi:10.1002/cpt.1889Siddiqi, Mehra, COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal, J Heart Lung Transplant,
doi:10.1016/j.healun.2020.03.012Smit, Ochomo, Aljayyoussi, Safety and mosquitocidal efficacy of high-dose ivermectin when co-administered with dihydroartemisinin-piperaquine in Kenyan adults with uncomplicated malaria (IVERMAL): a randomised, double-blind, placebo-controlled trial, Lancet Infect Dis,
doi:10.1016/S1473-3099(18)30163-4Tay, Fraser, Chan, Nuclear localization of dengue virus (DENV) 1-4 non-structural protein 5: protection against all 4 DENV serotypes by the inhibitor Ivermectin, Antiviral Res,
doi:10.1016/j.antiviral.2013.06.002Wagstaff, Sivakumaran, Heaton, Harrich, Jans, Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochem J,
doi:10.1042/BJ20120150DOI record:
{
"DOI": "10.1001/jama.2021.3071",
"ISSN": [
"0098-7484"
],
"URL": "http://dx.doi.org/10.1001/jama.2021.3071",
"author": [
{
"affiliation": [
{
"name": "Centro de Estudios en Infectología Pediátrica, Cali, Colombia"
},
{
"name": "Department of Pediatrics, Universidad del Valle, Cali, Colombia"
},
{
"name": "Clínica Imbanaco, Cali, Colombia"
}
],
"family": "López-Medina",
"given": "Eduardo",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Centro de Estudios en Infectología Pediátrica, Cali, Colombia"
},
{
"name": "Department of Pediatrics, Universidad del Valle, Cali, Colombia"
}
],
"family": "López",
"given": "Pío",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pediatrics, Universidad del Valle, Cali, Colombia"
},
{
"name": "State Health Department, Valle del Cauca, Colombia"
}
],
"family": "Hurtado",
"given": "Isabel C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Public Health, Universidad Icesi, Cali, Colombia"
}
],
"family": "Dávalos",
"given": "Diana M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clínica Imbanaco, Cali, Colombia"
},
{
"name": "POHEMA (Pediatric Oncologist and Hematologist) Foundation, Cali, Colombia"
},
{
"name": "Cali’s Cancer Population-based Registry, Cali, Colombia"
}
],
"family": "Ramirez",
"given": "Oscar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Universidad del Valle, Cali, Colombia"
},
{
"name": "Christus Sinergia Salud, Cali, Colombia"
}
],
"family": "Martínez",
"given": "Ernesto",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Neurólogos de Occidente, Cali, Colombia"
}
],
"family": "Díazgranados",
"given": "Jesus A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clínica Imbanaco, Cali, Colombia"
},
{
"name": "Department of Internal Medicine, Universidad del Valle, Cali, Colombia"
},
{
"name": "Clínica de Occidente, Cali, Colombia"
}
],
"family": "Oñate",
"given": "José M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Municipal Health Department, Cali, Colombia"
}
],
"family": "Chavarriaga",
"given": "Hector",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Caucaseco Scientific Research Center, Malaria Vaccine and Drug Development Center, Cali, Colombia"
}
],
"family": "Herrera",
"given": "Sócrates",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Microbiology, Universidad del Valle, Cali, Colombia"
}
],
"family": "Parra",
"given": "Beatriz",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Microbiology, Universidad del Valle, Cali, Colombia"
}
],
"family": "Libreros",
"given": "Gerardo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hemato Oncólogos, Cali, Colombia"
}
],
"family": "Jaramillo",
"given": "Roberto",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hemato Oncólogos, Cali, Colombia"
}
],
"family": "Avendaño",
"given": "Ana C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Health Experts Committee, Valle del Cauca, Colombia"
}
],
"family": "Toro",
"given": "Dilian F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Municipal Health Department, Cali, Colombia"
}
],
"family": "Torres",
"given": "Miyerlandi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "State Health Department, Valle del Cauca, Colombia"
}
],
"family": "Lesmes",
"given": "Maria C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Centro Médico Santuario, Cali, Colombia"
}
],
"family": "Rios",
"given": "Carlos A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clínica Imbanaco, Cali, Colombia"
}
],
"family": "Caicedo",
"given": "Isabella",
"sequence": "additional"
}
],
"container-title": "JAMA",
"container-title-short": "JAMA",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
3,
4
]
],
"date-time": "2021-03-04T17:30:28Z",
"timestamp": 1614879028000
},
"deposited": {
"date-parts": [
[
2021,
4,
14
]
],
"date-time": "2021-04-14T03:08:45Z",
"timestamp": 1618369725000
},
"indexed": {
"date-parts": [
[
2024,
4,
8
]
],
"date-time": "2024-04-08T17:20:37Z",
"timestamp": 1712596837821
},
"is-referenced-by-count": 260,
"issue": "14",
"issued": {
"date-parts": [
[
2021,
4,
13
]
]
},
"journal-issue": {
"issue": "14",
"published-print": {
"date-parts": [
[
2021,
4,
13
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jama/articlepdf/2777389/jama_lpezmedina_2021_oi_210022_1618250230.41706.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "1426",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2021,
4,
13
]
]
},
"published-print": {
"date-parts": [
[
2021,
4,
13
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1016/j.ijantimicag.2007.08.023",
"article-title": "Ivermectin: 25 years and still going strong.",
"author": "Omura",
"doi-asserted-by": "publisher",
"first-page": "91",
"issue": "2",
"journal-title": "Int J Antimicrob Agents",
"key": "joi210022r1",
"volume": "31",
"year": "2008"
},
{
"DOI": "10.1016/j.antiviral.2020.104760",
"article-title": "The broad spectrum antiviral ivermectin targets the host nuclear transport importin a/ß1 heterodimer.",
"author": "Yang",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Res",
"key": "joi210022r2",
"volume": "177",
"year": "2020"
},
{
"DOI": "10.1042/BJ20120150",
"article-title": "Ivermectin is a specific inhibitor of importin a/ß-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus.",
"author": "Wagstaff",
"doi-asserted-by": "publisher",
"first-page": "851",
"issue": "3",
"journal-title": "Biochem J",
"key": "joi210022r3",
"volume": "443",
"year": "2012"
},
{
"DOI": "10.1093/jac/dks147",
"article-title": "Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug.",
"author": "Mastrangelo",
"doi-asserted-by": "publisher",
"first-page": "1884",
"issue": "8",
"journal-title": "J Antimicrob Chemother",
"key": "joi210022r4",
"volume": "67",
"year": "2012"
},
{
"DOI": "10.1016/j.antiviral.2013.06.002",
"article-title": "Nuclear localization of dengue virus (DENV) 1-4 non-structural protein 5: protection against all 4 DENV serotypes by the inhibitor Ivermectin.",
"author": "Tay",
"doi-asserted-by": "publisher",
"first-page": "301",
"issue": "3",
"journal-title": "Antiviral Res",
"key": "joi210022r5",
"volume": "99",
"year": "2013"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro.",
"author": "Caly",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Res",
"key": "joi210022r8",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1016/j.onehlt.2020.100148",
"article-title": "COVID-19 and the rush for self-medication and self-dosing with ivermectin: a word of caution.",
"author": "Molento",
"doi-asserted-by": "crossref",
"journal-title": "One Health",
"key": "joi210022r14",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/j.healun.2020.03.012",
"article-title": "COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal.",
"author": "Siddiqi",
"doi-asserted-by": "publisher",
"first-page": "405",
"issue": "5",
"journal-title": "J Heart Lung Transplant",
"key": "joi210022r15",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.1093/pubmed/fdaa070",
"article-title": "Racial demographics and COVID-19 confirmed cases and deaths: a correlational analysis of 2886 US counties.",
"author": "Mahajan",
"doi-asserted-by": "publisher",
"first-page": "445",
"issue": "3",
"journal-title": "J Public Health (Oxf)",
"key": "joi210022r16",
"volume": "42",
"year": "2020"
},
{
"DOI": "10.1001/jamainternmed.2020.3857",
"article-title": "Assessment of COVID-19 hospitalizations by race/ethnicity in 12 states.",
"author": "Karaca-Mandic",
"doi-asserted-by": "publisher",
"first-page": "131",
"issue": "1",
"journal-title": "JAMA Intern Med",
"key": "joi210022r17",
"volume": "181",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19.",
"author": "Cao",
"doi-asserted-by": "publisher",
"first-page": "1787",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "joi210022r18",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of COVID-19: final report.",
"author": "Beigel",
"doi-asserted-by": "publisher",
"first-page": "1813",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "joi210022r19",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.16349",
"article-title": "Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial.",
"author": "Spinner",
"doi-asserted-by": "publisher",
"first-page": "1048",
"issue": "11",
"journal-title": "JAMA",
"key": "joi210022r20",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72?314 cases from the Chinese Center for Disease Control and Prevention.",
"author": "Wu",
"doi-asserted-by": "publisher",
"first-page": "1239",
"issue": "13",
"journal-title": "JAMA",
"key": "joi210022r23",
"volume": "323",
"year": "2020"
},
{
"article-title": "Hydroxychloroquine for early treatment of adults with mild COVID-19: a randomized-controlled trial.",
"author": "Mitjà",
"journal-title": "Clin Infect Dis",
"key": "joi210022r24",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2020.104805",
"article-title": "Ivermectin and COVID-19: a report in antiviral research, widespread interest, an FDA warning, two letters to the editor and the authors’ responses.",
"author": "Bray",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Res",
"key": "joi210022r25",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1080/13102818.2020.1775118",
"article-title": "Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: antiviral levels are not likely attainable with known dosing regimens.",
"author": "Momekov",
"doi-asserted-by": "publisher",
"first-page": "469",
"journal-title": "Biotechnol Biotechnol Equipment",
"key": "joi210022r26",
"volume": "34",
"year": "2020"
},
{
"article-title": "Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ICON Study.",
"author": "Rajter",
"journal-title": "Chest",
"key": "joi210022r27"
},
{
"DOI": "10.1002/cpt.v108.4",
"article-title": "The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19.",
"author": "Schmith",
"doi-asserted-by": "publisher",
"first-page": "762",
"issue": "4",
"journal-title": "Clin Pharmacol Ther",
"key": "joi210022r32",
"volume": "108",
"year": "2020"
},
{
"article-title": "Ivermectin as an adjunct in the treatment of refractory epilepsy [article in Spanish].",
"author": "Diazgranados-Sanchez",
"first-page": "303",
"issue": "7",
"journal-title": "Rev Neurol",
"key": "joi210022r33",
"volume": "65",
"year": "2017"
},
{
"DOI": "10.1016/S1473-3099(18)30163-4",
"article-title": "Safety and mosquitocidal efficacy of high-dose ivermectin when co-administered with dihydroartemisinin-piperaquine in Kenyan adults with uncomplicated malaria (IVERMAL): a randomised, double-blind, placebo-controlled trial.",
"author": "Smit",
"doi-asserted-by": "publisher",
"first-page": "615",
"issue": "6",
"journal-title": "Lancet Infect Dis",
"key": "joi210022r34",
"volume": "18",
"year": "2018"
},
{
"key": "joi210022r6",
"unstructured": "Frontline Covid-19 Critical Care Alliance. Accessed December 19, 2020. https://covid19criticalcare.com/"
},
{
"key": "joi210022r7",
"unstructured": "US Senate Committee on Homeland Security & Governmental Affairs. Early outpatient treatment: an essential part of a COVID-19 solution, part II. Accessed December 19, 2020. https://www.hsgac.senate.gov/early-outpatient-treatment-an-essential-part-of-a-covid-19-solution-part-ii"
},
{
"DOI": "10.1101/2020.11.21.392639",
"doi-asserted-by": "crossref",
"key": "joi210022r9",
"unstructured": "de Melo? GD, Lazarini? F, Larrous? F, . Anti-COVID-19 efficacy of ivermectin in the golden hamster.? bioRxiv. Preprint posted November 22, 2020. doi:10.1101/2020.11.21.392639"
},
{
"DOI": "10.1101/2020.11.02.363242",
"doi-asserted-by": "crossref",
"key": "joi210022r10",
"unstructured": "Arévalo? A, Pagotto? R, Pórfido? J, . Ivermectin reduces coronavirus infection in vivo: a mouse experimental model.? bioRxiv. Preprint posted November 2, 2020. doi:10.1101/2020.11.02.363242?"
},
{
"key": "joi210022r11",
"unstructured": "Ministerio de Salud, República del Perú. Resolución ministerial No. 270-2020-MINSA. Accessed December 19, 2020. https://cdn.www.gob.pe/uploads/document/file/694719/RM_270-2020-MINSA.PDF"
},
{
"key": "joi210022r12",
"unstructured": "Rodriguez Mega? E. Latin America’s embrace of an unproven COVID treatment is hindering drug trials. Nature. Accessed December 19, 2020. https://www.nature.com/articles/d41586-020-02958-2"
},
{
"key": "joi210022r13",
"unstructured": "Ministerio de Salud, Gobierno del Estado de Bolivia. Resolución ministerial No. 0259. Accessed December 19, 2020. https://www.minsalud.gob.bo/component/jdownloads/?task=download.send&id=425&catid=27&m=0&Itemid=646"
},
{
"key": "joi210022r21",
"unstructured": "World Health Organization. WHO R&D blueprint: novel coronavirus: COVID-19 therapeutic trial synopsis. Accessed December 20, 2020. https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf"
},
{
"key": "joi210022r22",
"unstructured": "US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Published November 27, 2017. Accessed December 20, 2020. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf"
},
{
"DOI": "10.21203/rs.3.rs-100956/v1",
"doi-asserted-by": "crossref",
"key": "joi210022r28",
"unstructured": "Ahmed? E, Hany? B, Abo Youssef? S, ? Efficacy and safety of ivermectin for treatment and prophylaxis of COVID-19 pandemic.? Research Square. Preprint posted November 17, 2020. doi:10.21203/rs.3.rs-100956/v1"
},
{
"DOI": "10.1101/2020.10.26.20219345",
"doi-asserted-by": "crossref",
"key": "joi210022r29",
"unstructured": "Hashim? HA, Maulood? MF, Rasheed? AM, Fatak? DF, Kabah? KK, Abdulamir? AS. Controlled randomized clinical trial on using ivermectin with doxycycline for treating COVID-19 patients in Baghdad, Iraq.? medRxiv. Preprint posted October 27, 2020. doi:10.1101/2020.10.26.20219345"
},
{
"DOI": "10.21203/rs.3.rs-109670/v1",
"doi-asserted-by": "crossref",
"key": "joi210022r30",
"unstructured": "Niaee? MS, Gheibi? N, Namdar? P, . Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: a randomized multi-center clinical trial.? Research Square. Preprint posted November 24, 2020. doi:10.21203/rs.3.rs-109670/v1"
},
{
"key": "joi210022r31",
"unstructured": "Clinical Trial of Ivermectin Plus Doxycycline for the Treatment of Confirmed Covid-19 Infection. Accessed December 21, 2020. https://clinicaltrials.gov/ct2/show/results/NCT04523831"
}
],
"reference-count": 34,
"references-count": 34,
"relation": {
"has-review": [
{
"asserted-by": "object",
"id": "10.3410/f.739669574.793584506",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jama/fullarticle/2777389"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [
"A Randomized Clinical Trial"
],
"title": "Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19",
"type": "journal-article",
"volume": "325"
}